OPERATIONS UPDATE: 1/27/26 BWS, Inc. is back to full operations. Please contact us with any questions or concerns about your scheduled pick up - (410) 437-6590 or customercare@bwaste.com
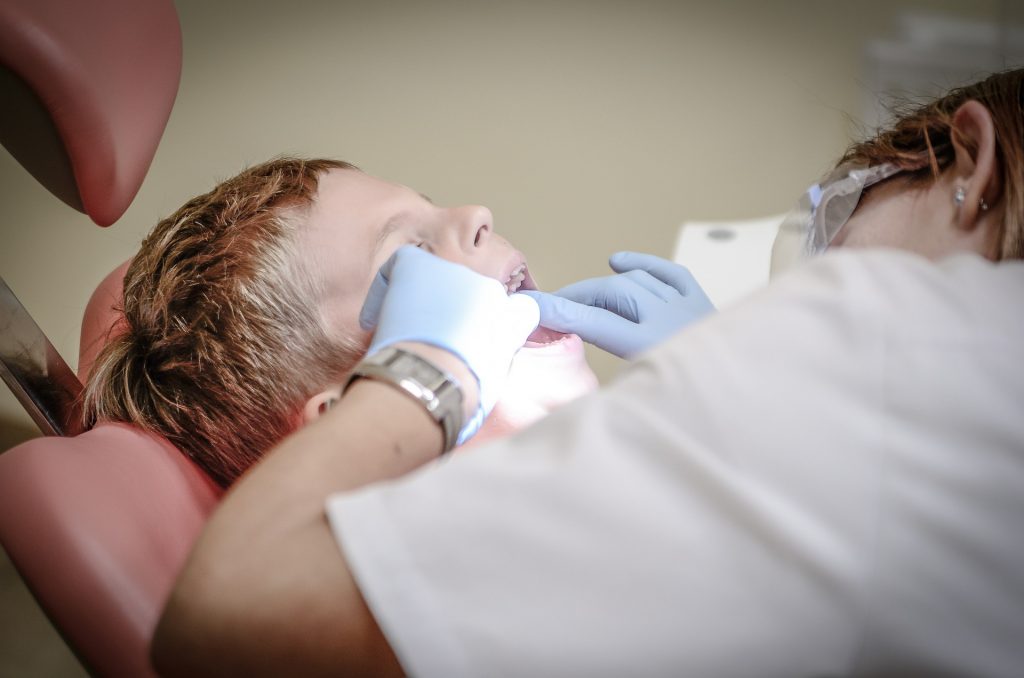
Understanding Dental Waste
Waste materials are routinely generated during the provision of oral healthcare in Dentists offices in Maryland and around the world. Most of this waste is nonhazardous and can be managed in the same way as household waste. However, some products used in dental practices can pose a risk to humans or the environment if discarded into landfills or poured down drains. These types of waste are regulated and must therefore be managed separately. Typically, two types of regulated waste are generated as a result of dental care: regulated medical waste, dental amalgam waste and hazardous chemical waste. These two types of waste each necessitate their own procedures and have different requirements for labeling, storage, disposal, and record-keeping.
Sharps
Contaminated disposable sharps (like needles) comprise the majority of regulated medical waste in dental practices. Proper storage and labeling of regulated medical waste is critical. Sharps containers must be color-coded or labeled, closable, leakproof on the sides and bottom, and placed as close as feasible to the location where the sharps are in use. OSHA requires that sharps be discarded as soon as possible after use, meaning that in most cases, the sharps container should be in the treatment room so that used needles, blades, wires, and other disposable sharps are not left until the end of the appointment to be discarded. Sharps containers should be capped when the contents reach the fill line and then discarded according to local regulations.
Medical Waste
Contaminated medical waste is generated as a product of healthcare and oral-health services when disposable items such as gauze are contaminated with blood, saliva, or other body fluids and when used sharp items such as needles and blades are discarded. The US Occupational Safety and Health Administration (OSHA) defines regulated medical waste in the Blood-borne Pathogens Standard. Regulated medical waste is liquid or semi-liquid blood or other potentially infectious materials (OPIMs), or items that would release the same if compressed. It also includes items that are caked with dried blood or OPIM that could be released during handling. Contaminated sharps and pathologic and microbiologic wastes that contain blood or OPIM are also regulated. All other waste, such as gloves, patient bibs, and disposable gauze that does not release blood, is not regulated but should be disposed and discarded in a manner that will not lead to accidental bare-handed contact by office or cleaning personnel.
Hazardous Dental Waste
Regulated hazardous waste includes products that are flammable, corrosive, toxic, or reactive, or pose some other risk to health or the environment. Hazardous waste can enter the environment either through solid waste sent to landfills or sanitary sewer systems when products are sent down the drain in the dental office. Hazardous material may enter the environment as a byproduct of dental procedures, such as when amalgam restorations are removed and high-speed suction lines carry the material to the wastewater stream. It may also enter the environment after use when disposing leftover or expired products that contain hazardous chemicals. Dental offices generate amalgam waste, which contains small traces of mercury, and various other hazardous and dangerous waste. The ADA encourages dental offices to recycle this waste appropriately. BWS provides the products and services to safely and efficiently dispose of dental amalgam waste. If your dental office is in need of waste removal management contact BWS for a free consultation. Check out our dental amalgam store for products and services.
Ready to get started?
We offer a variety of competitively priced service options with no contracts or hidden fees. Request a free quote.
Our Dedication to Providing the Highest Quality Regulated Waste Services
is Shown in What Our Clients Say About Our Work
We provide an outstanding value and service to our regulated waste customers and pride ourselves over the last 50 years on our 100% customer satisfaction with 99% customer retention ratio.
Paul R Miller, D.D.S. and Team
ZytoGen Patient Services
Akbar Masood, D.D.S.
Chris Donaldson
Dharani Jasthi
Tomicka Jackson-George, D.M.D.
Louis Sachs, D.D.S.
Kate Blair English
Dave Ryan
Kerry Owens, Avalon Plastic Surgery
Jamie Fleming, National Spine & Pain Centers
Keith Roberts, Biomatrix Specialty Pharmacy
Mireia Pedragosa Marin, Synergy Research
Rebecca Crouse, Randolph Macon Academy
Melissa C, Patriot Chiropractic
Dr. Rosario Ignacio
Dr. Thomas Lutz, Odenton Family Dentistry
Dr. Louis Sachs, DDS
Eric B.
Brenda Shah
Stay connected - Be the first to know!
Get BWS news and promotions straight to your inbox
Don't worry. We won't sell your information!