OPERATIONS UPDATE: 1/27/26 BWS, Inc. is back to full operations. Please contact us with any questions or concerns about your scheduled pick up - (410) 437-6590 or customercare@bwaste.com
Types of Chemotherapy Waste and Related Disposal Regulations
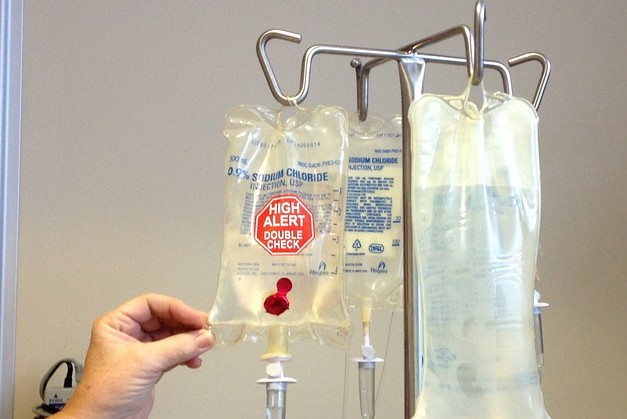
Out of all the medical procedures, chemotherapy generates some of the most hazardous waste in the medical field. They may not be infections, and therefore don't normally present risk to the general population, but they are highly toxic and corrosive! If disposed of improperly, these wastes may cause irreparable damage to natural resources. It's easy to get confused in all the terminology and regulations, which is why our Maryland chemotherapy waste disposal experts are here to offer you some guidance.
RCRA Hazardous Waste
The Resource Conservation and Recovery Act (RCRA) was enacted by the EPA to mandate procedures for disposal of wastes that are deemed hazardous. There are 4 lists of substances that are specifically listed as hazardous under the RCRA: F-list (non-specific source wastes), K-list (source-specific wastes), and P-list and U-list (discarded chemical products). When it comes to chemotherapy, you will likely have to deal with P-list and U-list substances such as cyclophosphamide, daunomycin or melphalan.
Characteristic Hazardous Waste
It's close to impossible to put together and maintain the complete list of all hazardous substances on the planet, even for a large organization such as the EPA. That's why the EPA has narrowed down the characteristics of a typical hazardous substance. These characteristics include toxicity, ignitability, corrosivity and reactivity. As a medical waste generator, it's one of your responsibilities to identify these “characteristic hazardous wastes” and manage them accordingly. All hazardous wastes need to be separated, properly packaged and labeled, as well as disposed of in the black waste container per EPA's mandate.
Trace vs Bulk Chemotherapy Waste
You may hear the words “trace” and “bulk” used to describe chemotherapy waste. Although these are not official EPA designations, these terms are widely used in the industry. Trace chemotherapy waste usually includes vials, bags, IV tubes and other items that used to contain chemotherapy drugs, but now qualify as “RCRA empty.” A container that used to hold a P-listed substance is “RCRA empty” only after it's triple-rinsed. A container that used to hold a U-listed substance is “RCRA empty” when there is less than 3% of the former volume left. If the applicable criteria are met, then this waste can be considered trace waste and can be managed as regulated medical waste and disposed of in yellow containers. Otherwise, it should be managed as hazardous waste. Bulk chemotherapy waste usually includes items that used to contain chemotherapy agents and that don't qualify as “RCRA empty.” Other types of bulk waste include materials used to clean up chemo spills and visibly contaminated PPE. Keep in mind that the bags of chemotherapy drugs that were unused but are out of date aren't necessarily hazardous waste. A substance becomes waste when it can no longer be reused, and expired drugs can sometimes be returned to the manufacturer through a “take-back” program.
Controlled Substances
Powerful painkillers, such as morphine or hydrocodone may be administered on chemotherapy patients while they are in your care. When disposing of these and other pharmaceutical wastage, be sure to check whether these drugs are listed as controlled substances per one of the DEA's schedules. While the DEA doesn't specify any particular packaging to be used for collecting these controlled substances, it does have specific regulations in regard to their disposal. If your facility works with controlled substances on a regular basis, you are probably already registered with the DEA. Disposal procedures may differ between DEA registrants and non-registrants, so check with the agency or with your medical waste disposal provider if you have any questions. Have any other questions about chemotherapy waste disposal? Feel free to contact BWS and we'll be happy to offer professional guidance.
Ready to get started?
We offer a variety of competitively priced service options with no contracts or hidden fees. Request a free quote.
Our Dedication to Providing the Highest Quality Regulated Waste Services
is Shown in What Our Clients Say About Our Work
We provide an outstanding value and service to our regulated waste customers and pride ourselves over the last 50 years on our 100% customer satisfaction with 99% customer retention ratio.
Paul R Miller, D.D.S. and Team
ZytoGen Patient Services
Akbar Masood, D.D.S.
Chris Donaldson
Dharani Jasthi
Tomicka Jackson-George, D.M.D.
Louis Sachs, D.D.S.
Kate Blair English
Dave Ryan
Kerry Owens, Avalon Plastic Surgery
Jamie Fleming, National Spine & Pain Centers
Keith Roberts, Biomatrix Specialty Pharmacy
Mireia Pedragosa Marin, Synergy Research
Rebecca Crouse, Randolph Macon Academy
Melissa C, Patriot Chiropractic
Dr. Rosario Ignacio
Dr. Thomas Lutz, Odenton Family Dentistry
Dr. Louis Sachs, DDS
Eric B.
Brenda Shah
Stay connected - Be the first to know!
Get BWS news and promotions straight to your inbox
Don't worry. We won't sell your information!




